- Investigation of the structure and properties of new inorganic and organic functional materials
- Investigation of structural and magnetic properties of materials under extreme conditions
- Real-time neutron investigations of irreversible transient processes in functional alloys
- Computer simulation of the structure and properties of new functional materials and nanosystems
A.M. Balagurov, D.P. Kozlenko, S.I. Tiutiunnikov (VBLHEP)
FLNP
S.E. Kichanov, V.A. Turchenko, A.I. Beskrovnyi, B.N. Savenko, E.B. Askerov, N.O. Golosova, M.L. Kraus, E.V. Lukin, G.M. Mironova, E.P. Popov, N.Yu. Samoilova, V.V. Sikolenko, S.V. Sumnikov
LIT
V.B. Zlokazov
VBLHEP
V.N. Shalyapin, V.V. Efimov, Yu.S. Kovalev, A.V. Rogachev, N.I. Zamyatin, I.A. Kryachko, V.A. Artyukh
Progress in modern condensed matter physics inextricably concerns the search and investigation of new materials with certain functional properties. Physical phenomena significant in the applied aspect, such as high temperature superconductivity, ferroelectricity, magnetism, luminescence, phase transitions and others have been discovered in various types of materials: complex oxides, amorphous compounds, glasses and composites. A wide range of physical properties and phenomena observed in these materials is often due to both certain peculiarities of the crystal and magnetic structure and to local structural inhomogeneities produced at the nanostructural and microstructural levels. Thorough structural investigations allow to determine the correlation between the functional properties of materials and the peculiarities of the structural configuration. This is essential both for the development of modern fundamental concepts describing the mechanisms of the observed physical phenomena and for improvement of the procedures aimed at the synthesis of new materials with desired properties for the development of various technologies.
To date, the most efficient technique for investigating the mutual arrangement of atoms in a substance is the diffraction of microparticles. The nature of neutron interactions with matter determines a range of advantages of neutron diffraction over the corresponding X-ray techniques in structural investigations. The high sensitivity of neutrons to the positions of light elements (H, Li, O) and elements with similar atomic numbers allows to obtain precise information about the crystalline structure of matter that is especially significant in conditions of structural disorder. The occurrence of a neutron's own magnetic moment allows to study the magnetic structure of materials. Thus, the neutron diffraction technique allows to carry out a complete analysis of both the crystal structure of rather complex materials and their magnetic structure simultaneously. Also, a significant factor in external influences is the high penetrating ability of neutrons that provides wide opportunities for working with temperature measurement devices on the sample (cryostats, furnaces).
Numerous investigations of various types of functional materials are implemented using neutron scattering techniques at the facilities of the IBR-2 reactor.
Currently, one of the most relevant areas in condensed matter physics and materials science is the investigation of low-dimensional layered van der Waals magnets, since such substances are essentially magnetic analogs of graphene, a unique two-dimensional material, the discovery of which was awarded the Nobel Prize in Physics in 2010. Recent investigations have shown that magnetic ordering in two-dimensional van der Waals magnets can be preserved at sufficiently high temperatures up to the monolayer limit. In addition, a large variety of new physical phenomena have been discovered in these materials when changing thermodynamic parameters (temperature and pressure), including superconductivity, topological spin excitations, skyrmion states, dielectric-metal transition and spin crossover.
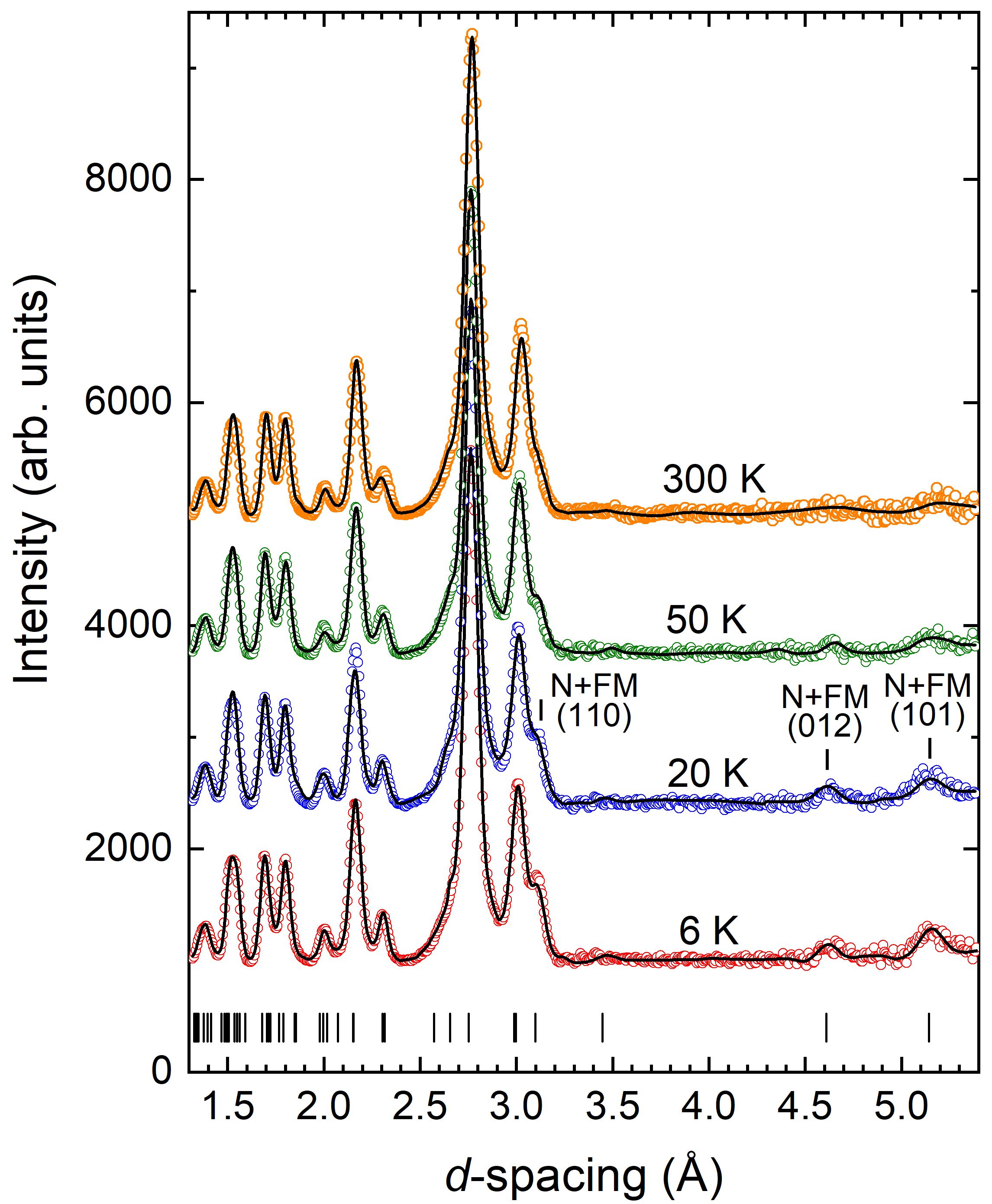
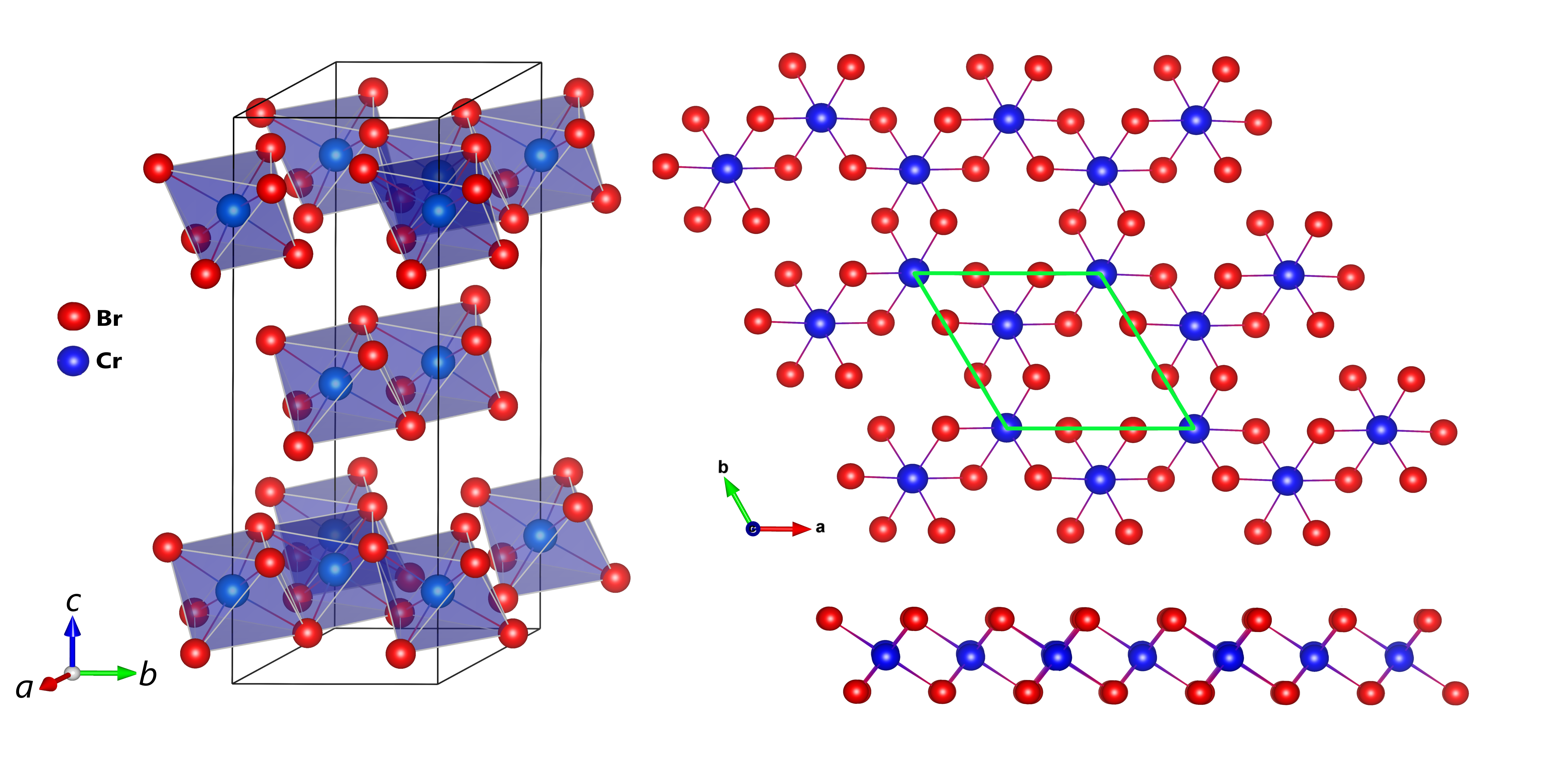
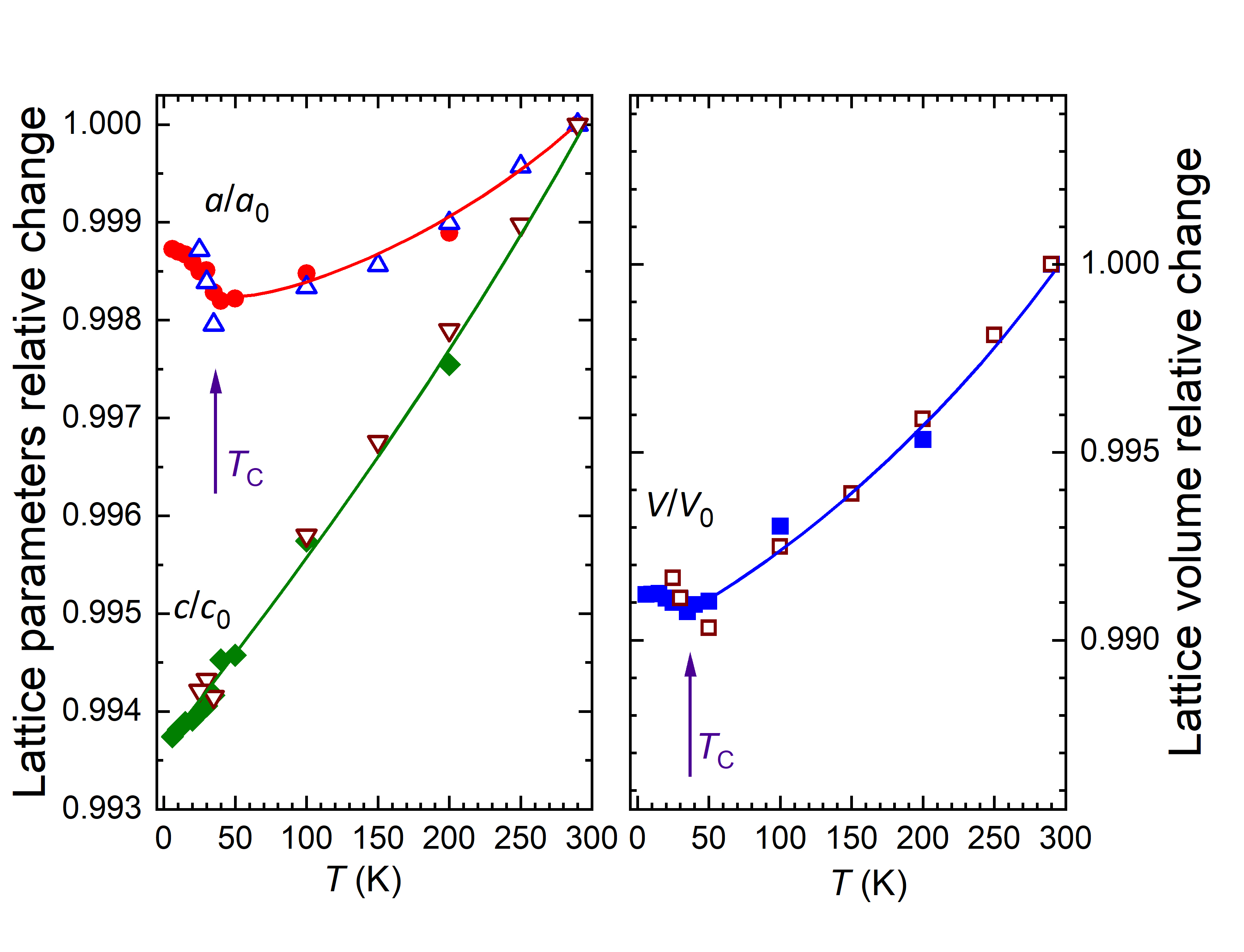
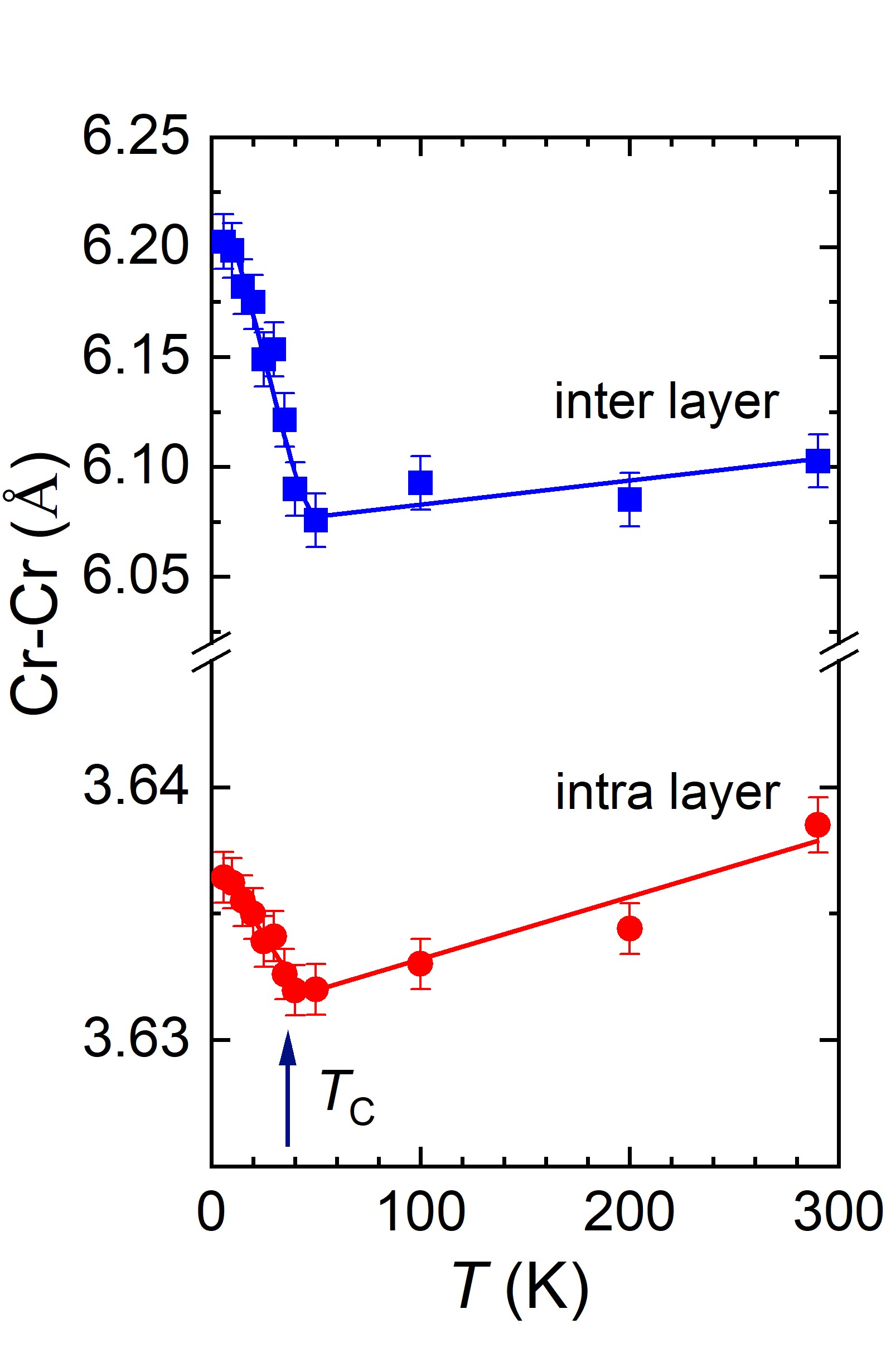
Investigations of the structural and magnetic properties of the CrBr3 compound using neutron diffraction [1] carried out at the JINR basic facility the IBR-2 pulsed reactor have allowed to detect unusual effects: the anomalous behavior of structural characteristics in the temperature range of ferromagnetic ordering of Tc and the negative thermal expansion of the volume of the crystal lattice and quasi-two-dimensional van der Waals layers in the temperature range T < Tc. It should be noted that negative thermal expansion is a relatively rare physical effect discovered only in a few classes of materials. Whereas, in most crystalline substances an increase in the volume of the crystal lattice and particular interatomic distances with increasing temperature (positive thermal expansion) is observed, in exceptional cases, as for CrBr3, in a certain range of thermodynamic parameters, the opposite effect, that is negative thermal expansion can be observed when the volume and interatomic distances decrease with increasing temperature (Fig. 1). It is interesting to note that graphene also shows negative thermal expansion and the coefficient of linear thermal expansion of atomic layers in CrBr3 in the range of T < Tc, αl = -1.6×10-5 K-1 is close to the corresponding value for graphene at low temperatures. The result obtained indicates good compatibility of materials such as CrX3 and graphene with respect to the prospects for developing heterostructures based on them, the practical use of which can be an essential step in the development of advanced generation of devices for spintronics, nanoelectronics, data registration and storage.
Fig.1. a) Neutron diffraction spectra of CrBr3 measured at various temperatures and profiles calculated using Rietveld refinement. b) Rhombohedral crystal structure of CrBr3 of R symmetry. Top and side view of the van der Waals atomic layers is shown on the right. c) Temperature dependences of the parameters and unit cell volume of the CrBr3 crystal lattice, relating to respective values at room temperature. d) Temperature dependences of distances between Cr magnetic ions inside van der Waals layers (intra-layer) and between layers (inter-layer).
Hexaferrites with the molar formula MFe12O19 (M = Ba, Sr or Pb) and solid solutions based on them draw attention due to the high values of Curie temperature (~732 K), relative ease of their preparation, high chemical stability and corrosion resistance [2–5]. These materials have been widely used in wireless communication systems, miniature devices, high density magnetic carriers and electronic components such as circulators, phase shifters, filters and induction coils. Strontium hexaferrites that possess high dielectric and magnetic losses in the microwave frequency range can be used as microwave absorption devices to reduce the impact of electronic interference from gigahertz electronic telecommunication systems.
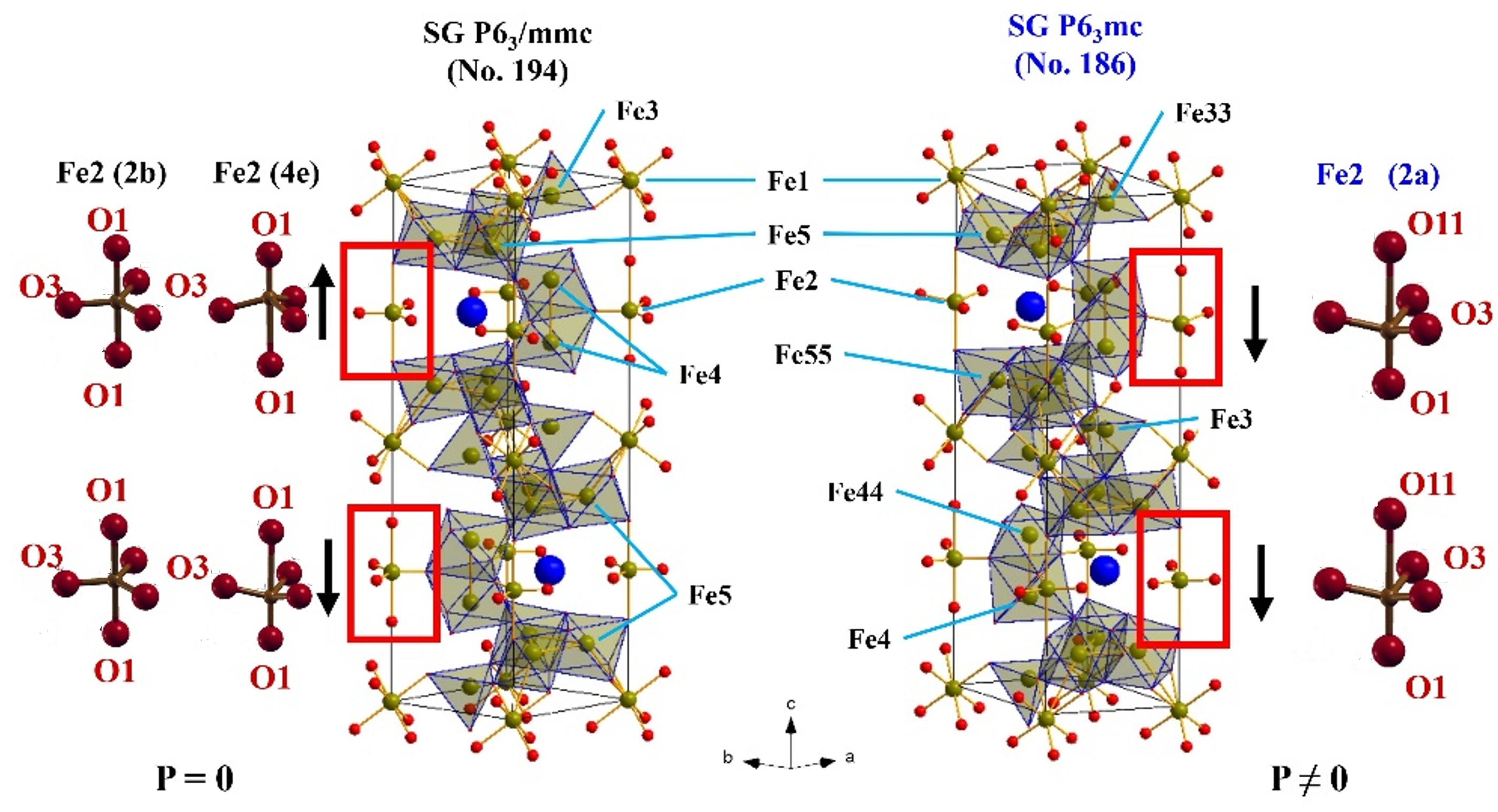
The parent hexaferrite MFe12O19 (M=Ba and Sr) is isostructural to magnetoplumbite PbO*6Fe2O3, the crystal structure of which was first defined by Adelskold in 1938. The unit cell contains two formula units (Z = 2) and includes 2 barium or strontium ions, 24 iron ions and 38 oxygen ions (Fig. 2, left) that develop a dense packing, as well as voids of several types: octahedrons (2a, 12k and 4fVI), tetrahedrons (4fIV) and trigonal bipyramids (2b) or (4e) with localized Fe ions in them.
Fig.2. Schematics of unit cells MFe12O19 (M= Ba; Sr; Pb, others) within centrosymmetric P63/mmc (No. 194) and non-centrosymmetric P63mc (No. 186) space groups.
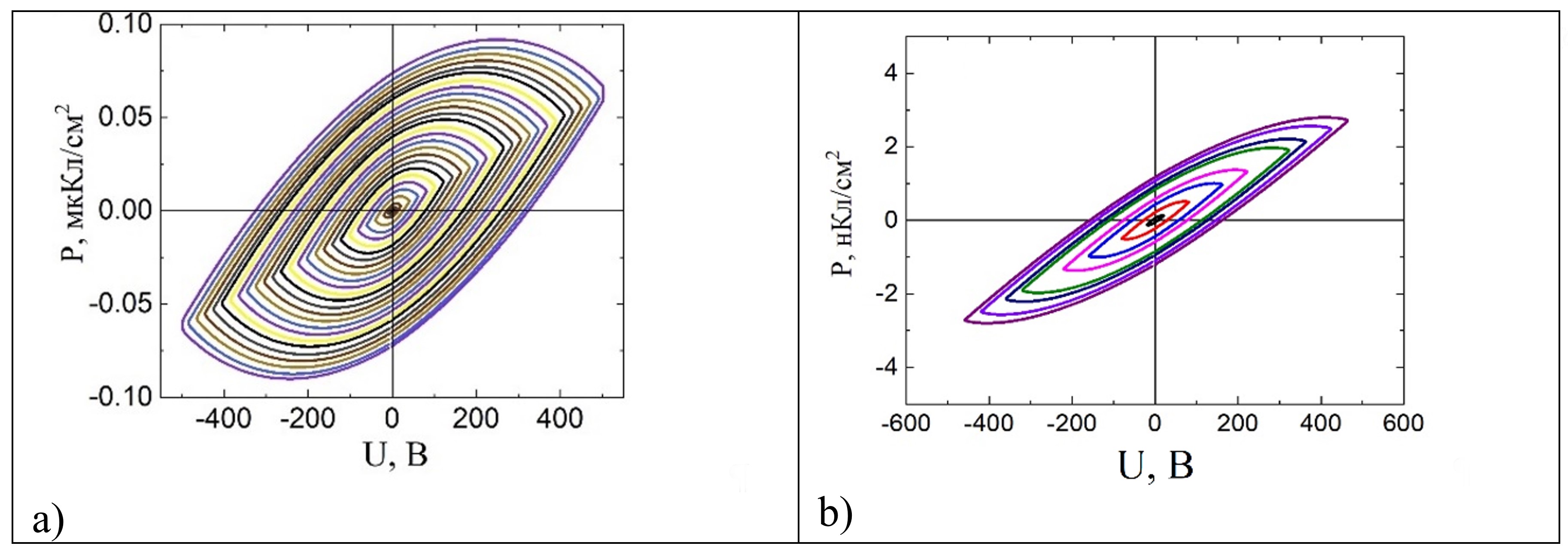
The occurrence of electric polarization (Fig. 3), observed in M-type hexaferrites, allows us to consider these materials as promising multiferroics.
Fig. 3. Polarization dependence of ceramic samples of BaFe12O19 and SrFe10.8In1.2O19 hexaferrites.
To explain the occurrence of residual electric polarization in the centrosymmetric structure of hexaferrites various assumptions are put forward, such as the occurrence of a noncollinear magnetic structure, distortion of a single oxygen octahedron, co-directional shift of iron ions in the positions of trigonal bipyramids, distortion of domain walls at the grain boundary.
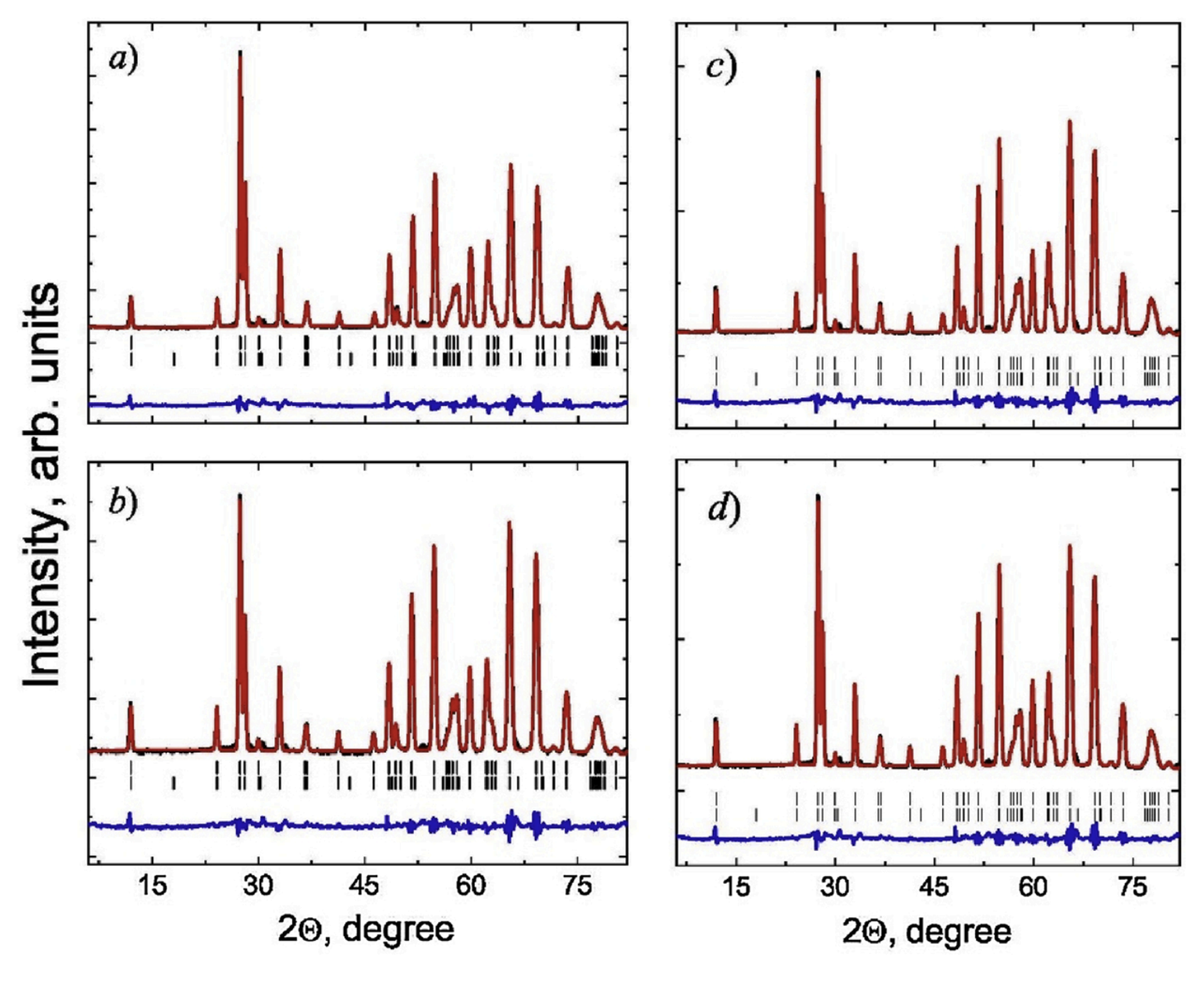
Despite many explanations, the true microscopic mechanism for the occurrence of ferroelectric properties in M-type hexaferrites is still not clarified. Previous investigations using neutron diffraction (Fig. 4) have shown the capability of describing the crystal structure of M-type barium and strontium hexaferrites with sufficiently high accuracy within both centrosymmetric P63/mmc (No. 194) and non-centrosymmetric P63mc (No. 186) (see Fig. 2, right) of the space group.
Fig. 4. Neutron diffraction patterns of the compound SrFe10.8In1.2O19 measured at temperatures: 1.5 K - (a) and (c), 300 K - (b) and (d), as well as refined using the Rietveld refinement within sp. gr. P63mc (No. 186) - (a) and (b), sp. gr. P63/mmc (No. 194) - (c) and (d).
Thus, the description of the crystal structure within the framework of the noncentrosymmetric sp. gr. P63mc allows to explain the co-existence of electric polarization and magnetic ordering in the structure of M-type hexaferrites as a result of disproportional distortion of oxygen polyhedrons and co-directional displacement of Fe ions in trigonal bipyramids.
Publications:
[1] Kozlenko D. P., Lis O. N., Kichanov S. E., et al., Spin-induced negative thermal expansion and spin–phonon coupling in van der Waals material CrBr3. npj Quantum Materials, 6(1), 1-5 (2021). doi:10.1038/s41535-021-00318-5
[2] V. Turchenko, V. G. Kostishin, S. Trukhanov, et al., Structural features, magnetic and ferroelectric properties of SrFe10.8In1.2O19 compound. Mater. Res. Bull, 138, 111236 (2021). doi:10.1016/j.materresbull.2021.111236
[3] V. Turchenko, V.G. Kostishyn, S.Trukhanov, et al., Crystal and magnetic structures, magnetic and ferroelectric properties of strontium ferrite partially substituted with in ions. Journal of Alloys and Compounds, 821, 153412 (2020). doi:10.1016/j.jallcom.2019.153412
[4] V. Turchenko, A. Trukhanov, S. Trukhanov, et al., Correlation of crystalline and magnetic structures of barium ferrites with dual ferroic properties. Journal of Magnetism and Magnetic Materials 477, 9-16 (2019). doi:10.1016/j.jmmm.2018.12.101
[5] V.A. Turchenko, A.M. Balagurov, S.V. Trukhanov, et al., Refinement of the Atomic and Magnetic Structures of Solid Solutions BaFe12 -xInxO19 (x = 0.1-1.2) by the Neutron Diffraction Method. Journal of Surface Investigation: X-ray, Synchrotron and Neutron Techniques, 13(1), 69-81 (2019). doi:10.1134/S1027451019010361